The document discusses GMP compliance audits. It defines GMP audits for a procedure to confirm that producers follow fantastic producing methods polices. There are two types of audits - onsite audits, which involve checking out the production website, and desktop audits, which evaluation documentation with no site stop by.Create detailed training p
The smart Trick of process validation in pharma That Nobody is Discussing
This study course will not likely deal with formulation growth, the regulatory submission processes or specific engineering models and affiliated qualification.Validation requires manufacturing several batches below defined parameters to ascertain regularity. Ordinarily, a few consecutive batches inside suitable boundaries reveal ample validation.
types of uv detectors hplc - An Overview
It will also be used to evaluate extremely reduced detection limitations of elemental and molecular factors, which isn't limited to framework identification.The cellular phase is evaporated and the column effluent is nebulized, similar to in an evaporative light-weight-scattering detector or even a mass spectrometer.Intuitive course of action to as
A Secret Weapon For method development
EMA Guideline on the requirements for the chemical and pharmaceutical good quality documentation about investigational medicinal products and solutions in scientific trialsWhat's more, a hollow multi-coil framework having a coaxial shut arrangement was utilized to assemble the supply coil structure instead of a standard electromagnet or lasting mag
A Review Of buy pharmaceutical documents
As soon as your software continues to be validated you are going to obtain an Bill so that you can come up with a payment for your remarkable volume. All invoices should be settled on receipt.Obtaining analyzed doc-connected worries, we identified which the staff hadn’t experienced appropriately-adjusted procedures with the collaborative perform
 Molly Ringwald Then & Now!
Molly Ringwald Then & Now! Heath Ledger Then & Now!
Heath Ledger Then & Now!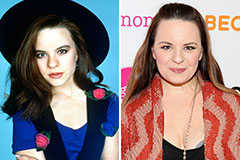 Jenna Von Oy Then & Now!
Jenna Von Oy Then & Now! James Van Der Beek Then & Now!
James Van Der Beek Then & Now! Sarah Michelle Gellar Then & Now!
Sarah Michelle Gellar Then & Now!